The Use and Optimisation of Catalysts for Automotive Fuel Cells
In the following paragraphs we will discuss fuel cell technology, as used in Fuel Cell Electric vehicles, and how optimised catalysts can improve their performance.
The Fuel Cell Electric Vehicle (FCEV) is a concept which has now been around for a long time. Whilst Battery Electric Vehicles (BEV) have received a lot of attention in the passenger vehicle market in recent years there is a growing interest in fuel cells for heavy duty vehicles and vehicles for longer range travel such as taxis and SUV’s. This interest, coupled with the emerging hydrogen economy (the global movement backed by many governments to switch from carbon emitting fossil fuels to hydrogen from zero or low carbon sources), means many OEM’s have active FCEV programs in place.
Fuel cell technology works alongside battery technology. A fuel cell electric vehicle uses a battery power train but utilises the electricity generated by the fuel cell to maintain the charge of the battery. Because of this a much smaller battery can be used. The fuel cell is fed with compressed hydrogen gas which passes over catalysts contained in the fuel cell stack generating electricity with only water emitted from the exhaust. When used with hydrogen generated by renewable resources (such as wind or solar), so called green hydrogen, there is zero carbon footprint. As the hydrogen can be refilled from a pump, the experience for the driver is similar to that of a diesel or petrol car. Short refill times at the pump rather than longer recharge periods at home or during inconvenient stops.
For heavy goods vehicles, fuel cells offer a significant reduction in weight when compared to battery electric vehicles, sacrifice less cargo space, and offer significantly longer ranges with the added benefit of these quick refill times making them a much more viable option.
It is expected that fuel cell trucks will have a lower total cost of ownership compared to battery electric trucks by 2025 and even be cost competitive with ICE trucks by 2030.
Fuel cells are largely based around Proton Exchange Membrane (PEM) technology which relies on catalysts, namely platinum and platinum alloys. It is the use of heterogeneous platinum group metal catalysts that allows PEM technology to give flexibility for automotive applications (such as rapid start up) and high levels of performance at ambient temperatures. Platinum is a rare metal and trades at relatively high prices. To mitigate this, platinum is used in finely dispersed form to maximize the surface to volume ratio. Today, the best Pt catalysts consist of 2-8 nm particles displaying a very large specific surface area. To prevent the sintering of these small nanoparticles, which can cause a decrease in catalytic activity during operation, the metal is often deposited on high surface area supports to prevent particle-particle contact. This also reduces the amount platinum required optimizing the use of the metal and limiting the impact on price.

When platinum or platinum alloys are deposited on supports, careful consideration must be taken when selecting the support type. The most widely used support is carbon. Carbon is widely available, cheap, stable in the conditions of the fuel cell and conductive. Originally something as simple as graphite would be used but now there are many highly technical grades of “carbon black” available.
When selecting the support material, the desired characteristics of the catalyst must be considered and there is a certain trade-off between high electrochemical performance and durability.
Above is an example showing the improved durability that Ames Goldsmith Ceimig’s HyPerTM FC 140 (40% platinum on a conductive carbon support) offers against a similar commercially available product.
Longer retention of electrochemical performance (durability) translates to a longer life for the fuel cell.
The improved performance of the Ceimig HyPerTM grades is a result of a two-year, cross company development program and is achieved through an improved deposition technique, specialist selection of carbon support materials and post production treatments. This product, HyperTM FC 140, uses a carbon support which has a specially selected for its uniform surface characteristics promoting durability.
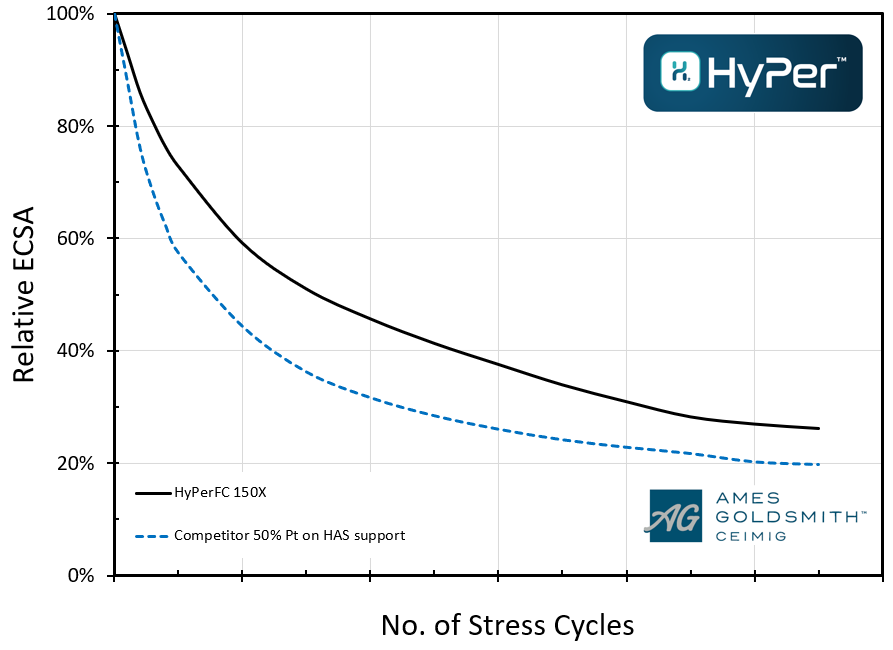
When opting for high electrochemical performance over durability a higher surface area carbon support with a highly structured surface is often chosen.
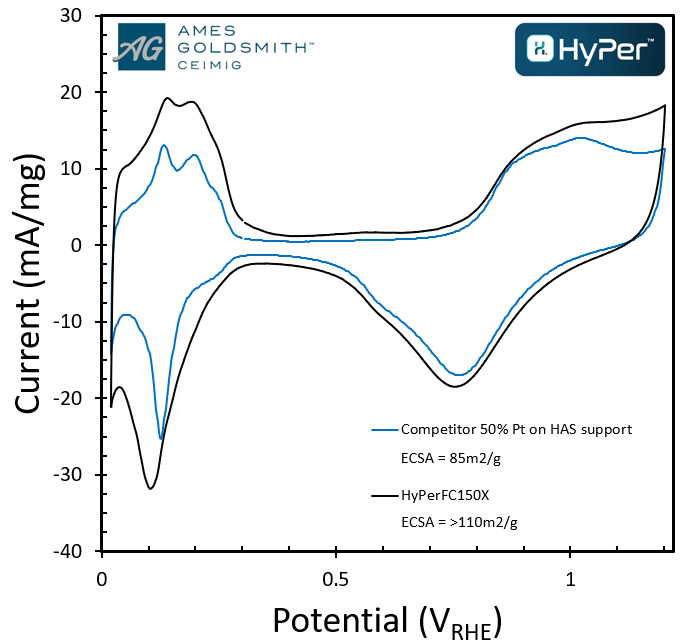
Above you can see a comparison of Ceimig’s HyPerTM FC 150X (50% platinum on an ultra-high surface area support) verses a similar commercial grade having a higher electrochemical surface area whilst below you can see the retention of this electrochemical performance through improved durability:
High electrochemical surface area can lead to greater power density for the same volume of catalyst or can allow the stack designer to use less catalyst for the same power. The increased durability means this level of performance lasts significantly longer.
In fuel cells the oxygen reduction reaction, which takes place at the cathode, is the most arduous reaction. This typically requires a higher loading of platinum to facilitate the reaction of hydrogen ions with atmospheric oxygen to reform water. The slightly easier hydrogen oxidation reaction, i.e., the splitting of hydrogen molecules into protons and electrons, taking place at the anode of the fuel cell can use lower platinum loadings. However, this catalyst layer can be prone to poisoning by carbon monoxide (temporary poisoning) and other substances such as sulphur (permanent poisoning) present in the incoming hydrogen gas.
Because of this, alloys of platinum with other materials may be used to further extend the life. Improvements have been shown by combining platinum with other metals. Alloys of cobalt and nickel are good examples used in the automotive industry offering synergistic effects and improved performance. Platinum can be combined with other PGM’s in either alloy or core-shell form to exploit beneficial effects. For example, platinum alloyed with ruthenium can prevent carbon monoxide poisoning of the catalyst. These synergistic effects can greatly enhance the performance and life of the fuel cell system. In high end automotive fuel cell systems iridium, or an iridium alloy, may be also used on the cathode in addition to platinum to function as a backflow catalyst in case of fuel starvation.
By selecting the correct catalyst in terms of support medium & loading the performance of the fuel cell can be greatly improved with higher power density and longer life.
Wayne Thornhill Sales Manager Ames Goldsmith Ceimig